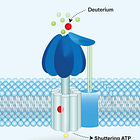
The ATPase 'Stutter'
Might mitochondrial deuterium content be a long-range communicator of environmental conditions?
Mitochondria appear to have evolved complex gating mechanisms that selectively deplete the deuterium, particularly where hydrogens are used to drive the rotation of the ATPase nano-rotors. Due to the unique kinetic isotope effects of deuterium, its activity inside these biological turbines diverges from its lighter counterparts. In the mid-2000s, Abdullah Olgun hypothesised in a seminal paper that deuterons at the site of an ATPase could cause a “stutter”, momentarily shifting the rotational movement of the rotor, and thus, the synthesis of ATP. Even though only trace amounts of deuterium nuclei (deuterons) seem capable of reaching the electron transport chain where they can be pumped against a chemical gradient, deuterium’s role here is likely not to be a trivial one.
“[E]volution seems to depend on deuterium-related regulatory processes via submolecular proton tunneling event (reaction) architectures, with coinciding significant isotope fractionation properties.”
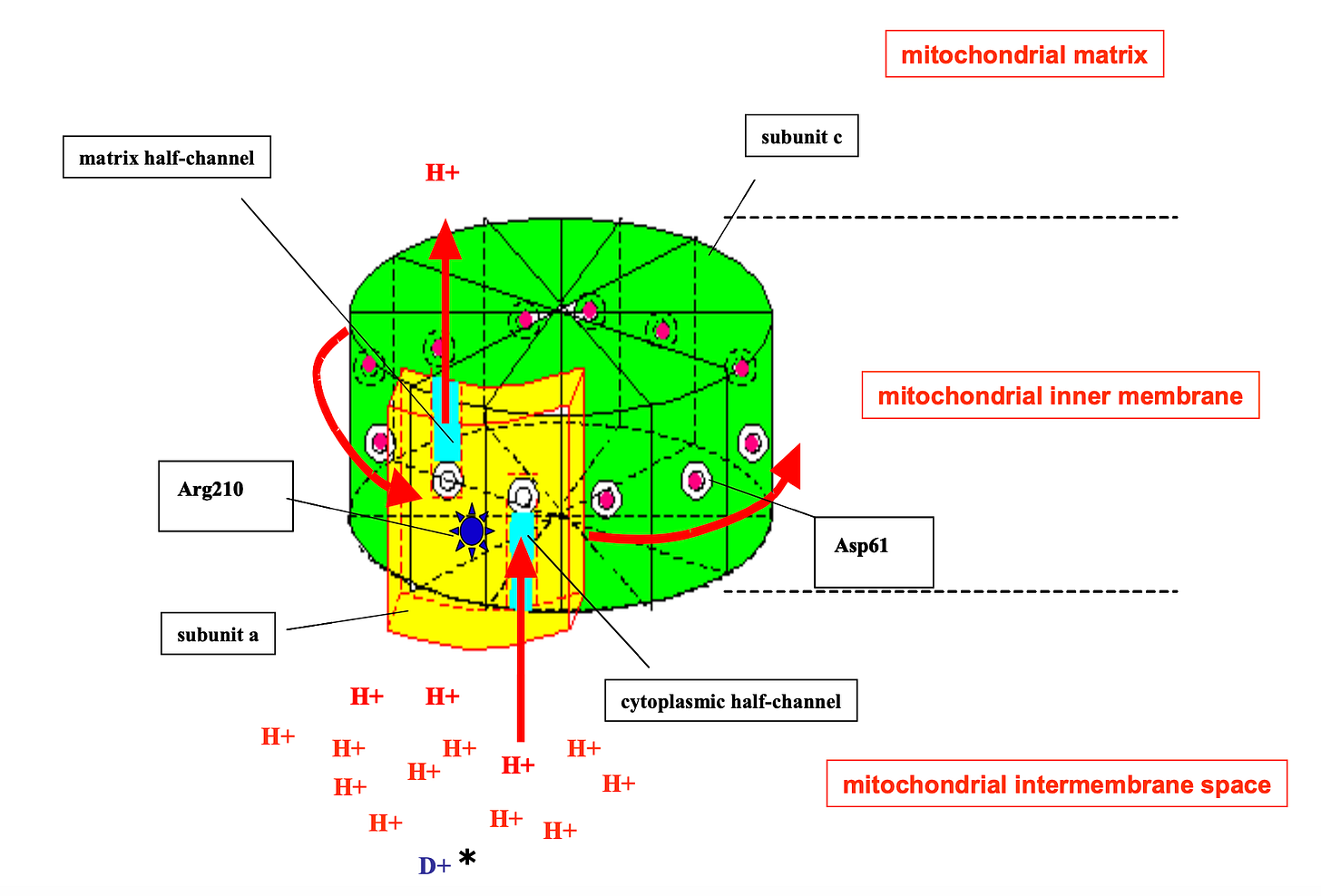
In my previous article on deuterium, I emphasised that deuterium should not be classified as “bad”, or even necessarily detrimental — even in the context of the mitochondria and the transformation of energy.1 The idea I am interested in exploring pertains to the deuterium abundance in the mitochondria being utilised as a signal, integrated throughout the entire mitochondrial network. With approximately 1021 ATPases in the body (±~1 to 2 orders of magnitude), even modest shifts in the abundance of deuterium to hydrogen could hypothetically have tangible physiological effects.
“This slow dissociation [of a deuteron] may cause temporary stutter in the rotor. If we were able to observe all ~15000 ATP synthases in a mitochondrion, we would see a percentage of them stuttering at any given time.”
The rapid rotation of the F₁ ATPase subunit and consequent movement of charged particles (H+ or D+) generate relatively large electromagnetic fields and bioelectric gradients that are modulated by the speed of rotation. These fields propagate well beyond the nano-rotor, likely influencing the surrounding water and behaviour of charged particles like H+, e- and ROS. It may also be that these ATPase-driven fields contribute to long-range communications and non-chemical signalling. The frequency of ATPase ‘stuttering’ defined by the relative abundance of deuterons in the mitochondria affects both bioelectricity and endogenous electromagnetic fields — a mechanism by which environmental signals (eg., D/H ratio) can be integrated systematically.
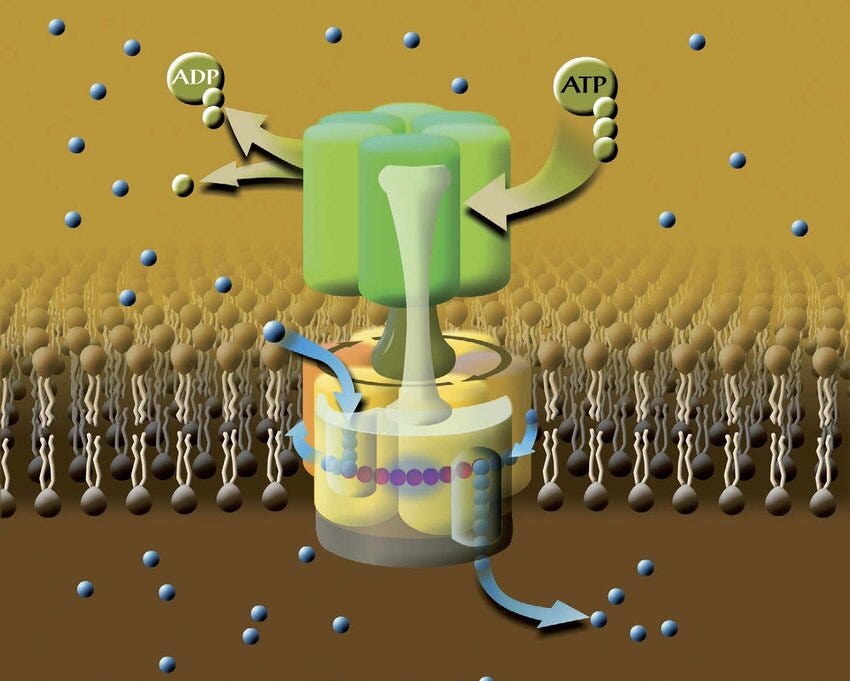
My hypothesis is that the relative abundance of deuterium to hydrogen in the mitochondria shapes the electromagnetic and bioelectric conditions. In this case, more stuttering would correspond to an equatorial environment where more deuterium is available when compared to more polar regions. This might suggest that more ATPase stuttering could be beneficial when coupled with stable diurnal cycles, lower geomagnetic field strength, higher UV availability and warmer average temperatures — other signals of equatorial regions.
So rather than being a priori detrimental, deuterium’s influence on ATPase nano-rotors could hypothetically transduce meaningful environmental information through the frequency of stuttering. Analogous to how information can be transferred by a sequence of beeps like in morse code, the ATPase stutter frequency (defined by the relative abundance of deuterium to hydrogen in the mitochondria) may confer meaningful information via shifts in endogenous electromagnetic fields and bioelectric phenomena. When coupled appropriately, such shifts could potentially scale up to tangible physiological changes, although admittedly this is drawing a long bow.
While this hypothesis is entirely speculative, it would account for the integration of locale-specific environmental conditions2 in a system-wide manner. What is particularly appealing about an idea like this is that it builds a framework where nuance is invited — where deuterium is as biologically relevant as hydrogen, a heterarchical system, rather than a hierarchical one. Although this is merely one facet of how the deuterium to hydrogen ratio plays a role in regulating biological function, it seems quite fundamental given how life is, in some sense, the movement of charges.
Summary
A widely cited theoretical paper from 2007 posits that deuterons’ (D+) behaviour diverges from that of hydrogen in ATPase nano-rotors, causing a “stutter” due to its unique kinetic isotope effect.
The rotation of the ATPase complexes generate electromagnetic fields consistent with their rapid movement of charged hydrogen nuclei — fields that can theoretically propagate meaningful information beyond the cite of the electron transport chain.
D+, while gated from the mitochondria to a certain extent, will find its way into the electron transport chain in trace amounts, causing these stuttering effects. Although excess dietary deuterium is an ever-present problem today, its presence in the diet is expected, and should play an important physiological and regulatory role.
It is possible that the stutter frequency (defined by the relative presence of D+ in the mitochondria) is an integral aspect of non-chemical communication, and the “stutter” is not ‘bad’, so to speak, but carries information that, when integrated with other appropriate signals, facilitates adaptation.
Related Articles
Related Podcasts
I should make explicit, however, that I mean this in the context of food and water intake that is locale-appropriate and not artificial by means of food importation or processing.
In this specific context, the D/H ratio would be primarily modulated by the type of foods available (location and season) and the macronutrient distribution therein; as fats are depleted of deuterium relative to proteins, and proteins depleted relative to carbohydrates.