Thermodynamics is essentially the study of energy. Energy takes many forms, some of which we are acutely aware of such as sound energy, thermal energy and light (electromagnetic energy). There are ‘laws’ that govern the relationships between different forms of energy. The one I want to focus on here is the second law of thermodynamics.
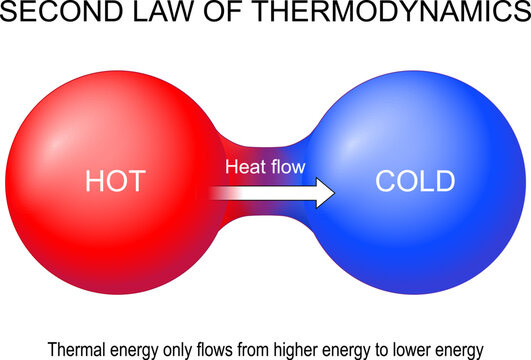
“The second law of thermodynamics asserts that heat cannot move from a reservoir of lower temperature to a reservoir of higher temperature in a cyclic process.”
- Encyclopaedia Britannica
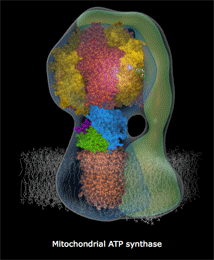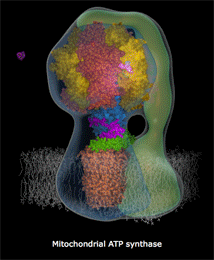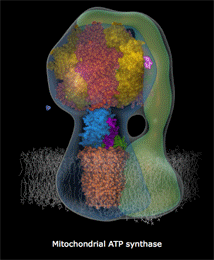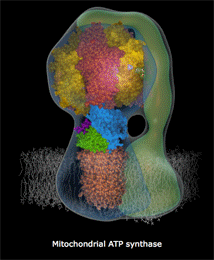
Another way to think about this law is that energetic systems gravitate towards an equilibrated state. This is the law that promotes the idea of a gradual ‘heat death’ of the universe as eventually hot-spots of energy will all dissipate and the universe will approach and attain a homogenous state of thermodynamic equilibrium. It is also analogous to the idea of a proton motive force that drives the synthesis of ATP in the mitochondria. By generating a charge gradient over a selectively permeable membrane, the mitochondria and use this natural tendency for charges to equilibrate to spin the ATPase rotor at 7800rpm.
This idea is vital, that energetic systems seek thermodynamic equilibrium; uniformity. Although the stability of physical laws have at times been questioned with good cause, these Newtonian rules have been hugely successful and have ultimately driven the advances of the industrialised world.